The COVID 19 pandemic in Thailand over the past three years has afforded us the chance to observe the capacity of Chulalongkorn university, Chulalongkorn hospital, and the Thai Red Cross to assist Thailand in coping with a global health crisis.In addition to location management and medical personnel, skills, technologies, and innovation have been implemented to care for Thais in accordance with international standards and World Health Organization (WHO) recommendations. However, the virus is constantly evolving. The demand for COVID-19 vaccines is escalating rapidly; consequently, global citizens would facilitate the development by spreading news of a series of successes that are the result of the skills and contributions of researchers from all over the world. “ChulaCov19” is one of these contributions, made by The Center of Excellence in Vaccine Research and Development or Chula Vaccine Research Center (Chula VRC), Chulalongkorn University’s Faculty of Medicine.

As the success of mRNA COVID-19 vaccine, “ChulaCov19” in trials in monkey (from collaboration between University of Pennsylvania and Chulalongkorn University in 2020), the project is waiting for government’s permission to advance to human trials towards the end of 2020
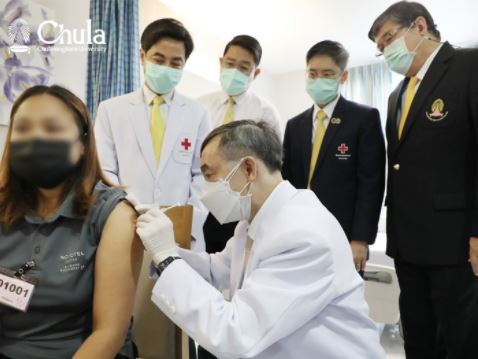
The first Thai-made mRNA vaccine, same type of Pfizer and Moderna, has begun its human trial on 14th June 2021 by vaccinate volunteers in phase 1 and phase 2 to see immune response towards vaccine ChulaCov19, this process was done from the help of Chulalongkorn hospital, Thai Red Cross, Faculty of Medicine, Chulalongkorn university, and The Chula VRC, Faculty of Medicine, Chulalongkorn university.
The results of phase 2 of the US-made ChulaCov19 vaccine showed that the vaccine was safe and had side effects similar to Pfizer’s. It can boost antibody and cellular immunity 2.5 times more than the Pfizer/BNT vaccination. It inhibits cross-species of COVID-19’s Alpha, Beta, Gamma, and Delta strains. Omicron strains have decreased but are still higher than Pfizer/BNT. Since Pfizer/BNT suggests a third vaccination to boost the immune system, the third vaccination against Omicron strains in the ChulaCov19 vaccine will be tested.

Development and production of the ChulaCov19 mRNA vaccine on a large scale in Thailand by Bionet Asia (lot BNA159), a Thai company located in Phra Nakhon Si Ayutthaya Province, has been completed. Thailand can now manufacture the first mRNA ChulaCov19 vaccine (lot BNA159) for testing on approximately 20,000 volunteers. Bionet Asia (BNA, a Thai company) is able to develop mRNA vaccine production faster than anticipated. Bionet Asia is one of only ten vaccine factories in the world capable of producing mRNA vaccines, alongside Pfizer and Moderna. The properties and quality of vaccines produced in Thailand were sent to England for testing and were found to be comparable to those produced in the United States. The establishment of a partnership between the Chula VRC and the Thai Private Vaccine Factory is regarded as a crucial link in the chain for achieving future epidemic-problem sustainability. If a new epidemic or mutated infection occurs, Thailand will be able to invent, develop, and produce mRNA vaccines to address the country’s crisis more rapidly. The goal is to facilitate the export of vaccines used to stop severe infectious diseases that will be a problem globally in the future. This includes vaccines for non-infectious diseases such as cancer and allergies.
The Faculty of Medicine, Chulalongkorn University has submitted documents related to vaccines to the Food and Drug Administration since the end of November 2021. It is still in the process of reviewing the documents.
Prof. Kiat Ruxrungtham, MD. the director of project said “The development of ChulaCov19 vaccine has been supported from the government, private, and public sector such as the national vaccine institute, the national research institute, Chulalongkorn universities, and well as donation from Alumni doctors network of Faculty of Medicine, Chulalongkorn university.

It is clear that the values of The Chula VRC, Faculty of Medicine, Chulalongkorn University “Discover, develop and deliver safe, effective and affordable vaccines” has been carried vigorously. It is evidently shown especially in other protective measures against other viruses such as the human immunodeficiency virus (HIV), Dengue, Leptospirosis, House dust mite allergies, all of which has been published with more than 19 products. Faculty of Pharmacy, Veterinarian, and Science, as well as other universities such as Chiangmai university, King Mongkut’s University of Technology Thonburi, and collaborations with other vaccine research institutes such as Vaccine Research Center (VRC) National Institute of Allergy and Infectious Diseases (NIAID) National Institute of Health (USA); Department of Adjuvant and Antigen Research, U.S., Military H.I.V. Vaccine Research Center (VRC) (WRAIR). It is no wonder that the vaccine creation process which usually takes more than 10 years, Chulalongkorn university could be able to get through such processes with haste.

BY
- Chula Vaccine Research Center (Chula VRC)
- Faculty of Medicine, Chulalongkorn University
Related articles:
- https://www.chula.ac.th/en/clipping/42518/
- https://www.chula.ac.th/news/43752/
- https://www.chula.ac.th/news/49656/
- https://www.chula.ac.th/news/52440/
- https://www.newswise.com/coronavirus/chula-medicine-announced-the-success-of-clinical-trials-for-the-chulacov19-vaccine-and-acceleration-of-the-next-phase-of-research/?article_id=756327
- https://www.bangkokpost.com/life/social-and-lifestyle/2132527/leading-by-example
Others
Getting Even with Inequality in Asia
“Getting even – Public policies addressing inequality in Asia”. This book is a collection of research papers drawing lessons from economic and social policies that help reducing inequality in selected Asian countries.