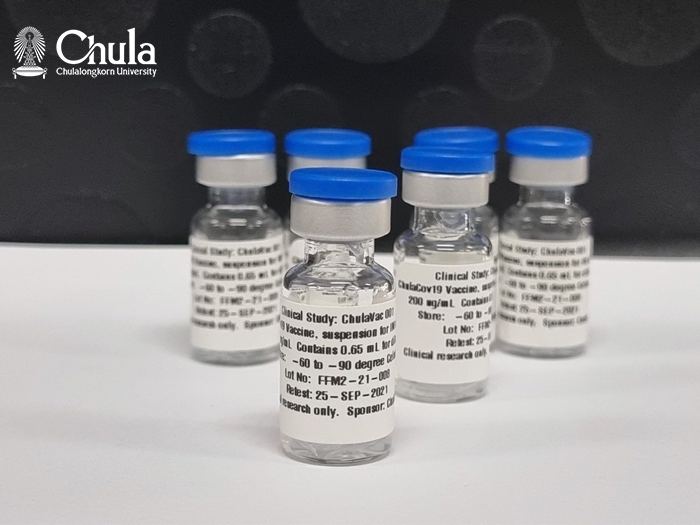
The situation of COVID 19 pandemic in Thailand in the past 2 years has given us an opportunity to see the capability of Chulalongkorn university, Chulalongkorn hospital, and the Thai Red Cross in providing aid to help Thailand in coping with global health crisis. Alongside with location management and medical personnels, the incorporation of skills, technologies, and innovation has been used to care for Thais adhering to the international standards and World Health Organization’s (WHO) guidelines. However, the virus is continuously evolving. The urge for COVID-19 vaccines are increasing rapidly; as a result, global citizens would facilitate the development through news of series of success that contributes from the skills and contribution of researchers all around the world. “ChulaCov19” is one of such contributions, contributed from The Center of Excellence in Vaccine Research and Development or Chula Vaccine Research Center (Chula VRC), Faculty of Medicine, Chulalongkorn University. https://www.chulavrc.org/
As the success of mRNA COVID-19 vaccine, “ChulaCov19” in trials in monkey (from collaboration between University of Pennsylvania and Chulalongkorn University in 2020), the project is waiting for government’s permission to advance to human trials towards the end of 2020
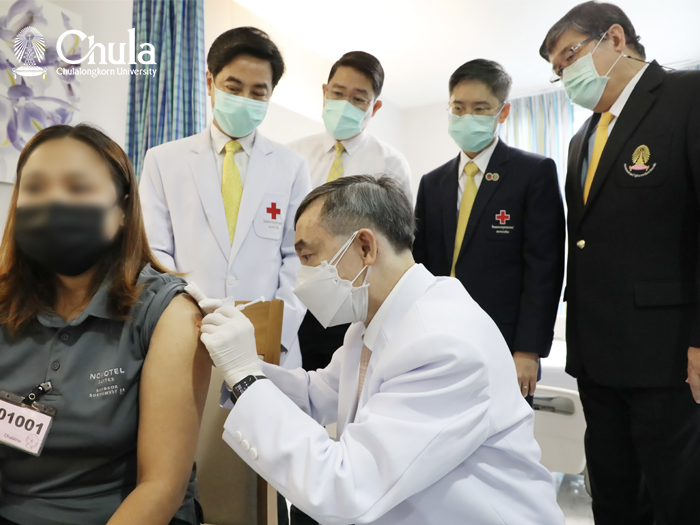
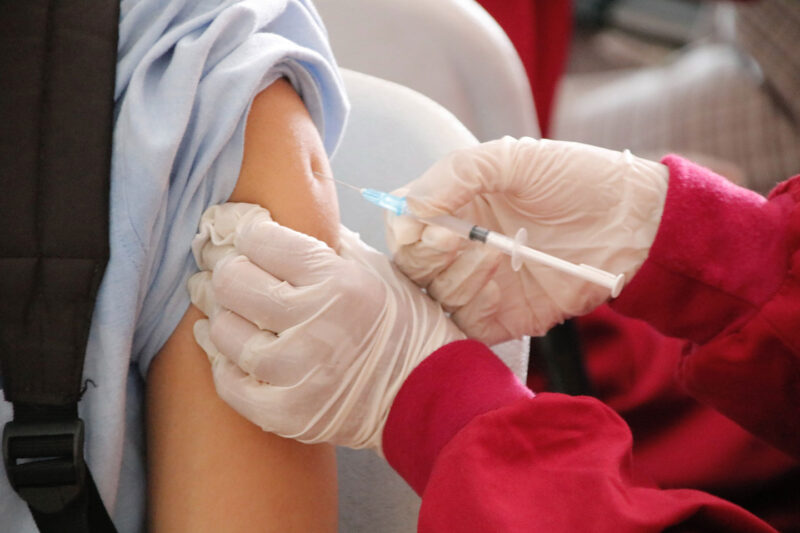
Recently, the first Thai-made mRNA vaccine, same type of Pfizer and Moderna, has begun its human trial in 14th June 2021 by vaccinate volunteers in phase 1 and phase 2 to see immune response towards vaccine ChulaCov19, this process was done from the help of Chulalongkorn hospital, Thai Red Cross, Faculty of Medicine, Chulalongkorn university, and The Chula VRC, Faculty of Medicine, Chulalongkorn university.

Prof. Suttipong Wacharasindhu, MD. the director of Chulalongkorn hospital said that “The Chula Vaccine Research Center, Faculty of Medicine, Chulalongkorn University is an institute that has been
collaborated by many medical researchers both on an domestic and global scale to research, innovate, and create vaccines to help protect citizens. The development of ChulaCov19, today is the first day to test volunteers in Phase 1 and Phase 2 under close facilitation from many fields as well as medical experts and doctors from the vaccine research institute to increase confidence in the safety measures of human vaccine trials.”
Prof. Kiat Ruxrungtham, MD. the director of project said “The development of ChulaCov19 vaccine has been supported from the government, private, and public sector such as the national vaccine institute, the national research institute, Chulalongkorn universities, and well as donation from Alumni doctors network of Faculty of Medicine, Chulalongkorn university.
It is clear that the values of The Chula VRC, Faculty of Medicine, Chulalongkorn University “Discover, develop and deliver safe, effective and affordable vaccines” has been carried vigorously. It is evidently shown especially in other protective measures against other viruses such as the human immunodeficiency virus (HIV), Dengue, Leptospirosis, House dust mite allergies, all of which has been published with more than 19 products. There are collaborations on many levels in the university from Faculty of Pharmacy, Veterinarian, Science, as well as to other universities such as Chiangmai university, King Mongkut’s University of Technology Thonburi, and collaborations with other vaccine research institutes such as Vaccine Research Center (VRC) National Institute of Allergy and Infectious Diseases (NIAID) National Institute of Health (USA); Department of Adjuvant and Antigen Research, U.S., Military HIV Research Program (MHRP) และ Walter Reed Army Institute of Research (WRAIR). It is no wonder that the vaccine creation process which usually takes more than 10 years, Chulalongkorn university could be able to get through such processes with haste.

BY
Chula Vaccine Research Center (Chula VRC)
Faculty of Medicine, Chulalongkorn University
Previous related articles:
http://www.sustainability.chula.ac.th/report/938/
Related articles:
- https://www.newswise.com/coronavirus/chula-medicine-announced-the-success-of-clinical-trials-for-the-chulacov19-vaccine-and-acceleration-of-the-next-phase-of-research/?article_id=756327
- https://www.bangkokpost.com/life/social-and-lifestyle/2132527/leading-by-example
- https://www.forbes.com/sites/carolineseydel/2020/12/09/thai-researcher-aims-to-make-his-country-a-vaccine-powerhouse/?sh=16e87394232f
- https://www.genengnews.com/covid-19-candidates/chulalongkorn-university-center-of-excellence-in-vaccine-research-and-development-chula-vrc-national-research-council-of-thailand-nrct-and-bionet-asia/
- https://www.ryt9.com/en/prg/244595
- http://www.chulavrc.org/
Others
The Development of the Creative Tourism Project
Creative tourism is an industry with high potential which can generate income and improve the quality of life nation-wide with sustainability.